MOVED – Visit Akron’s new blog
Our blog has moved!
Update your bookmarks and visit us at our new location: https://www.akronbiotech.com/about-us/news/
– The Akron Team
Akron Biotech at Cell and Gene Therapy World – All the Events
Cell and Gene Therapy World, organized by Phacilitate, is taking place this coming week. After multiple years in Washington, DC, the conference has moved to Miami, Florida, where from January 17 – 20, 2017 delegates will be able to interact with over 350 companies discussing the latest advances in the commercialization of cell and gene therapies.
The entire agenda can be accessed here.
Akron will attend and participate at Cell and Gene Therapy World through a number of panels, talks and our in-person booth.
Booth
Please stop by booth #114 to meet us and learn about our latest discoveries, products and technologies. We will be there for the entire duration of the conference, and welcome anyone to interact and see the innovations we will be presenting.
Panels and Talks
Akron will participate in a number of panels and talks.
Dr. Claudia Zylberberg, Akron’s CEO, will participate in the panel Examining the cutting edge in game-changing cryoformulation, cryopreservation, thawing and storage technologies and techniques in action. The panel takes place on January 18, 2017 at 12:45 – 1:30.
Dr. Sandro Matosevic, Senior Director of Research and Development, will present during the session titled Clinical vs. commercial logistics: preparing for and navigating the transition from clinical to commercial scale. The session is scheduled to take place between 4:45 – 6:00 PM on Wednesday, January 18, 2017. The session will include presentations, a panel discussion and an audience Q&A.
Meet Us
Alongside public appearances, Akron will be available to meet, on a one-on-one basis, any party interested in discussing potential collaborations, partnerships or any of our products or technologies. Please contact us directly.
We hope to see you at Cell & Gene Therapy World!
Scaffolds Promote the Development and Growth of Organoids
Organoids – miniature organs produced via stem cells which show three-dimensional, tissue-like arrangement – enable, through physiologically-relevant environments, the study of various pathologies which these structures are assembled for. Typically assembled devoid of scaffolds, organoids have been used for both drug discovery and as three-dimensional tissue engineered constructs. As tissue engineered assemblies, organoids have originally been formed on the basis of cultured stem cells, such as induced pluripotent stem cells, which rearrange, through spatially-restricted lineage committment, into layered structures.
In the last few years, studies have highlighted that organoids can be enhanced with the introduction of substrates to which cells adhere.
In 2016, a manuscript by Dr. Timothy O’Brien from the Stem Cell Institute at the University of Minnesota described the generation of cerebral organoids from human pluripotent stem cells with the support of a chemically defined hydrogel. They managed to achieve protein expression representative of forebrain, midbrain, and hindbrain development. The manuscript was published in Stem Cells Translational Medicine.
This was demonstrated again last week, when a new study appeared which described the development of scaffold-support human lung organoids. Authored by lab of Dr. Jason Spence at the University of Michigan Medical School, the manuscript, titled A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids, presented the development of microporous poly(lactide-co-glycolide) (PLG) scaffolds as substrated onto which human pluripotent stem cell-based organoids developed into maturate of lung epithelial tissue.
Upon transplanting scaffold-grown organoid tissue, airway-like epithelial tissue was observed. This was attributed to the enhanced support provided by the polymer-based support scaffolds.
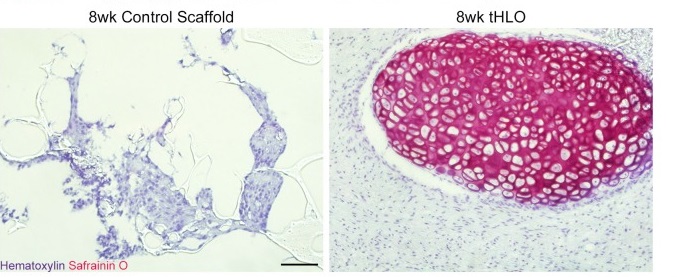
The authors argued that this study was the first demonstration of the development of hPSC-derived human lung organoids which showed robust engraftment in vivo and differentiation into an organized pseudostratified epithelium.
Organized cartilage was observed following Safranin0 stanining within the scaffold-human lung organoids following transplantation (Figure above), alongside cartilage marker SOX9, and the human mitochondrial marker huMITO. The scaffold-human lung organoid also displayed abundant vasculature within the tissue.
This manuscript, alongside previous other studies, forms a growing body of work which suggests that providing a physical environment to stem cell-based organoids, is critical in ensuring development and organization of mature tissue-like structure and function. Further developments may shed more light into the relationship between scaffold physicochemical properties and organoid-based human tissue establishment to develop more sophisticated system for organ regeneration.
Creating cellular function: Microparticles that act as heart stem cells developed
If the development of genetic editing approaches to induce functional changes to cell behavior to enhance their efficacy has led to remarkable advances in gene-engineered cell therapy, the development, on the other hand, of synthetic systems for tissue engineered and regenerative medicine-based therapies has made inroads as a robust, fully-controllable bottom-up approach to cell and gene therapy. Synthethic approaches have often offered complementary tools – such as three-dimensional scaffolds – upon which to build in vivo-like biological function. an example of this are tissue engineered-scaffolds and three-dimensional substrates.
Now, microengineered structures have been developed which cross over to fully replace cellular function, rather than enhance it.
Case in point: A new study described the development of microengineered particles with cardiac cell-like function, as a type of “synthetic stem cell.”
The work was described in a manuscript published last week in Nature Communications, titled Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome by the lab of Dr. Ken Che, associate professor of molecular biomedical sciences at North Carolina State University.
Called synthetic cell-mimicking microparticles, they are made up of poly (lactic-co-glycolic acid) (PLGA) to which cardiac cell-extracted growth factors are added. This creates, effectively, a biodegradable, synthetic shell which contains beneficial growth factors that impart biological function. Such biological function was tested both in vitro and in vivo, in a mouse model with myocardial infarction. In both cases, and particularly in vivo, the microparticles exhibited cardiac cell-like function to support cardiac tissue growth.
While biological function is successfully displayed by these structures, they do not exhibit other cellular-like activity, such as division.
Further work is expected to answer more questions about the long-term potential of these structures as therapeutic vehicles, which will bring such work closer to answering questions about its potential clinical benefit.
2016 at Akron Biotech: A year in review
Not much time is left before we close 2016 and enter the new year. As we wrap up the final few weeks of this year, we want to take the time-as we do every year-to reflect on the year that is ending.
2016 has been, for many reasons, a year of growth, excitement and surprises — globally for the cell and gene therapy field, it will be remembered for numerous breakthroughs both scientifically as well as clinically, alongside a reinvigorated federal interest in regenerative medicine and immunotherapy.
At Akron, 2016 brought all of that, alongside innovation – in discovery, manufacturing and our involvement in national and international consortia advancing the therapeutic possibilities of regenerative medicine.
Here, we want to reflect on some of Akron’s highlight from this past year:
- In the first quarter, Akron announced acceptance of a Drug Master File for Interleukin-2 by the FDA, and invites all parties interested in referencing it in their regulatory submissions to submit a request.
- At the beginning of the year, Sen. Tommy Baldwin presented The Advancing Standards in Regenerative Medicine Act, which calls for the establishment of a Standards Coordinating Body in Regenerative Medicine and Advanced Therapies. Akron is part of the task force behind this SBC, efforts regarding which are still ongoing.
- Our industry-leading product portfolio expanded by the introduction of innovative new products: we reformulated recombinant Eryothropoietin which now has enhanced stability and activity, and launched a 5% HSA solution.
- Our research efforts resulted in the publication of a number of manuscripts. A highlight is a review on liposome systems for drug delivery, Pharmaceutical Liposomal Drug Delivery: A Review of nEW Delivery Systems and a look at the regulatory landscape, which was published in Drug Delivery.
- Akron was awarded an SBIR Phase II grant by the Department of Defense for our leading innovations in the space of DMSO-free cryopreservation.
- Akron has always maintained leading presence at International Conferences and symposia. 2016 was no different: Akron was featured at ISCT, ISSCR, TERMIS, CAR-TCR to name a few.
As 2016 draws to a close, we look forward to 2017 and wish everyone happy holidays.
Our blog will resume in two weeks, on January 1st – we hope to see you usher in the new year with us.

Newly discovered ‘anti-CRISPR proteins’ can prevent CRISPR mistakes, new study says
CRISPR/CAS9 has emerged as a flexible and effective approach for genome editing with a promise for the correction of disease-causing mutations. However, most studies have so far demonstrated CRISPR as an on/off system, with little temporal control of its activity or function. Now, new research from the University of Toronto described the discover of a number of proteins that allow to precisely do this: control the activity of CRISPR specifically and conditionally, and at defined time points.
Published in Cell, the study is titled “Naturally Occurring Off-Switches for CRISPR-Cas9” from the lab of Dr. Alan Davidson.
The authors identified naturally occurring protein inhibitors of a CRISPR-Cas9 system. A bioinformatics-based approach allowed the authors to discover three proteins that inhibit N. meningitidis type II-C CRISPR-Cas system, by interacting with NmeCas9 to function as off-switches for NmeCas9 genome editing activity.

They further showed that members of all three anti-CRISPR families bind directly to the NmeCas9/sgRNA complex and inhibit in vitro DNA cleavage. Interestingly, they displayed unrelated sequences, leading the authors to postulate that they operate under different mechanisms as well.
Evolutionarily, Cas9-associating anti-CRISPRs were postulated to prevent the acquisition of new spacers (such as viral DNA) in response to external invasions, with CRISPR having a profound effect on horizontal gene transfer. This implication has profound effects on therapeutics being developed based on CRISPR/CAS9 technology.
Therapeutically, these proteins can prevent off-target effects and are thus exciting potential therapeutic targets.
The entire study can be read here.
FDA: “Stem cell-cased cures must be supported by sound science”
In September, the Food and Drug Administration held a public hearing on the NIH campus, wherein stakeholders – researchers, the general public and investors – were called upon to voice their opinions on the current status of stem cell-based therapies in the country.
Last week, the FDA followed this up with an opinion statement, via a paper in the New England Journal of Medicine, highlighting the FDA’s position of these therapies.
In the paper, FDA commissioner Robert Califf, alongside the Center for Biologics Evaluation and Research (CBER) Director Peter Marks and CBER Deputy Director Celia Witten urged caution. In doing so, the FDA warned against “unproven” treatments as well as those with “unsufficient data.”
The FDA claimed,
“To ensure that this emerging field fulfills its promise to patients, we must first understand its risks and benefits and develop therapeutic approaches based on sound science”
before adding,
“Often, these cells (whether derived from autologous or allogeneic sources) are being used in practice on the basis of minimal clinical evidence of safety or efficacy, sometimes with the claim that they constitute revolutionary treatments for various conditions.”
They go as far as calling the lack of data “worrisome,” by adding:
“Published data derived primarily from small, uncontrolled trials plus a few well-controlled, randomized trials have not reliably demonstrated the effectiveness of stem-cell treatments even in some of the most systematically studied conditions, such as heart failure and graft-versus-host disease”
The FDA calls both autologous and allogeneic treatments concerning, albeit for different reasons.
These claims will come as a disappointment to proponents of stem cell-based therapies, particularly patients who, absence other options, were hopeful that these cures would provide the benefit other medications were not able to.
The entire paper, titled “Clarifying stem-cell therapy’s benefits and risks,” can be read here.
Gene Therapy Toward More Control?
Last week, a study in Nature Communications by the lab of Dr. Ming Guo at the University of California, Los Angeles described a transgene-based approach to delete lethal mitochondrial DNA via genetic modifications which include expression of PINK1/parkin genes and other activity that promotes mitophagy. The implications of these results have significance for a number of severe diseases such as Parkinson’s and, more broadly, aging.
Such studies demonstrate the significant advancements that genetic engineering has made and the revolutionary potential of gene therapy. And with promising data coming out of clinical trials, coupled with novel gene editing approaches such as CRISPR/CAS-9, there is growing confidence in gene therapy as a viable therapeutic option in the future.
However, the area is still quite new and there are at least as many questions as there are answers. From a regulatory perspective, there is little that has been done to regulate gene editing. Currently, the UK is the only country that regulates mitochondrial replacement. While the FDA, in 2016, attempted to ignite discussion on the technology, further discussions were halted.
However, ongoing conversations are occurring. Regulatory authorities in the US are debating how to regulate these genetic modification technologies. The Human Gene Editing Initiative between the National Academies of Sciences and the National Academies of Medicine will release, in 2017, a Consensus Study on Human Gene Editing, the culmination of multiple years of works and public hearings, a consensus study on gene editing technologies which will cover not just the scientific merits of such technologies, but also the clinical, ethical, legal, and social implications of their use.
Interest in deeper regulation is also felt in the White House, with the President’s Council of Advisors on Science and Technology (PCAST) calling on the creation of a new entity tasked with developing a national biodefense strategy within six months, triggered by their argument that synthetic DNA, gene therapy, and genome-editing technologies like CRISPR open up security issues due to their ability to be misused.
It will be interesting to see how these initiatives shape up with the rapidly expanding scientific landscape.
Akron at World Stem Cell Summit Roundtable
Akron will participate at the Cell Therapy Manufacturing Roundtable at the upcoming World Stem Cell Summit, to take place December 6-9, 2016 in West Palm Beach, Florida. The roundtable will take place 1:30PM – 2:30PM on December 9th. Please let us know if you plan to attend and want to schedule a meeting.
See how a stem cell differentiates… up close and in 3D
The maturation of stem cells into various lineages has been studied extensively as the central feature of the therapeutic potential of stem cells in regenerative medicine. The commitment of a stem cells to a mature cell type is accompanied by epigenetic changes of the cell’s genetic makeup. Until now, studies of those events has uncovered a lot of information about the nature of the rearrangement of genes specific for the control of the cell’s specific state prior and during differentiation. The suggestion is that these rearrangements lead to 3D distribution of chromatin in vivo.
Now, advances in in high-resolution imaging have allowed for an unprecedented view into the 3D architecture of chromatin during cell maturation. A study, by the lab of Dr. Carolyn Larabell, from the Department of Anatomy at the University of California San Francisco and the Physical Biosciences Division at Lawrence Berkeley National Laboratory, reported on the use of soft X-ray tomography as a tool for the visualization of genetic changes during stem cell differentiation.
Soft x-ray tomography had previously been used to demonstrate high-resolution, 3D imaging of human stem cells, where it allowed the visualization of all major organelles at 50 nm spatial resolution.
Using the technique, the authors have been able to resolve the biophysical state and the conformation of the genome with high spatial precision during cell differentiation, feats that had been unachievable previously.
In the paper, titled “Soft X-Ray Tomography Reveals Gradual Chromatin Compaction and Reorganization during Neurogenesis In Vivo,” the authors imaged three cell types of the olfactory epithelium, horizontal basal cells (HBCs), globose basal cells (GBCs), and mature olfactory sensory neurons (mOSNs). Thesse cells were selected as they could be appropriated as multipotent stem cells, neuronal progenitors, and terminally differentiated neurons, respectively.
During differentiation, chromatin undergoes reorganization and compaction, moving from a multi-potent state to a differentiated, mature state. During those stages, the authors also observed the relocation of chromatin from the periphery of the nucleus, where it is mainly located in stem cells, to the nuclear core in mature neurons.
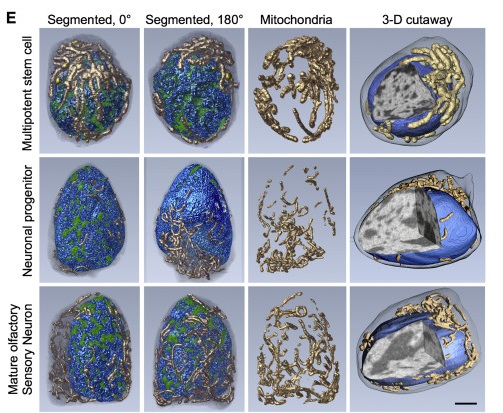
Beyond the remarkable high-resolution details, the study demonstrated that soft x-ray tomography is a powerful tool for the study of the genetic architecture during cellular differentiation in vivo.
Bacteria for gene therapy: FDA updates guidance on microbial vectors
Like other viral and non-viral gene delivery methods, biological properties of bacteria, particularly their tendency to naturally colonize tumor sites, have allowed them to be exploited for DNA delivery to cells or tissues. The concept of exploiting bacteria as gene delivery vectors has existed for some time, however the field is still new, particularly as mechanisms by which bacteria colonize tumor sites is still largely unknown.
With the field growing, the FDA has stepped in to update their previous guidances on microbial vectors for gene therapy by issuing an updated version which introduces more control over the manufacturing of such delivery vectors for therapeutic processes that are proceeding to IND submission and approval.
It must be mentioned that, in their current form, the guidances are intended to cover only initial IND submissions, not products in advanced stages.
In the updated guidelines, the FDA recommends that IND sponsors provide detailed information about product manufacturing and characterization of microbial vectors in their submission documents.
Thise guidances, “Recommendations for Microbial Vectors used for Gene Therapy,” include recommendations for both Manufacturing and Testing of the material. Specifically:
- Description of the MVGT bacterial strain, including its growth and storage conditions, the reagents used to grow it, and details about the bacterial and inserted genetic material.
- Description of how the MVGT Drug Product is produced, isolated, purified and formulated for administration to humans;
- Description of the product containers;
- Manufacturing process qualifications (such as current Good Manufacturing Processes (cGMP) requirements) for Phase 1, Phase 2 and Phase 3 clinical trials, as appropriate.
- Results from appropriate product testing for identity, purity, viability and potency at each stage of production, including safety, purity, potency, presence of DNA plasmids, viability, and cell number, stability, residual moisture content, and more.
- In vivo biological activity studies, safety and biodistribution profiles
In relation to this information, which significantly expands on previously published guidelines in terms of information required by IND sponsors and drug manufacturers, the FDA provides recommendations to ensure the safety in therapies where microbial vectors are used.
Of particular note is the FDA’s new focus on requiring data from in vivo studies. In vivo data must include, among others:
- Analysis whether the microbial vector for gene therapy (MVGT) can continue to replicate and spread in the body after treatment, including to non-target tissues, and
- MVGT activity and distribution with and without antibiotics to guide antibiotic use in human trials.
Additional guidance points include design of trials and selection of vector, to ensure appropriate data is collected and appropriate subjects and vectors are chosen to minimize risk and increase safety.
